Words & Pics: Dan Maslic
It doesn’t matter if you’re a race car driver, hot-rod builder, or a pilot, it helps to understand the theory of air pressure; specifically barometric pressure and its effects on the machines we build, drive, ride, sail and fly. Consider this: I take a jug of water (the 1-gallon size you can buy at any store) and ask you to hold it for me. You unenthusiastically say “yes” and you physically have no problem with it as it’s only a few pounds. We here in the USA would call one gallon of water 8.34 lbs to be exact. The Brits would argue that it’s 10 lbs, but we’ll stick with US gallons and call it 8.34 lbs. Now, I decide to ask you to hold another jug and I place it directly on top of the first one. I do the same again and place a third jug on top of the other two. At this point, you’re starting to feel the weight. You’re feeling the effects of pressure. The weight of the upper two jugs of water is bearing down on the jug at the bottom and that’s making the bottom jug feel heavy. If I put a scale between each jug of water one at a time to weigh the stack, the bottom jug will feel heaviest at roughly 25 lbs (3 jugs at 8.34 lbs each). Placing the scale under the middle jug would indicate a weight of roughly 16.7 lbs (2 jugs’ weight), and under the upper-most jug, the scale would read 8.34 lbs, the weight of just the one jug. In other words, the more you stack on top, the heavier the bottom jug feels. In between are varying amounts of weight depending on where you measure.
As you might imagine, barometric pressure is measured by an instrument called a barometer. It’s typically stated in units of “inches of Mercury” (inHg) or in “milli-bars” (mB). 1 inHg is equal to 33.86 mB. The idea of barometric pressure was put forth by a clever Frenchman back in the 1630’s or so, and later an equally clever Italian guy invented the first Barometer. The barometer measured air pressure by how high a column of mercury would rise in a tube with a vacuum on one side when acted on by ambient air on the other. We’re beyond that technology with modern sensors now (hats off to the French and Italians for getting us started) and we now use a pressure transducer to determine the current barometric pressure. The transducer converts the static air pressure into a proportional electrical voltage. This voltage is then read by a computer and used to determine the local air pressure. This is handy stuff: In airplanes, we use it to help us determine our operating altitude; in the automotive realm, it helps the EFI controller (the ECM/PCM) correctly calculate and model the engine airflow for fuel and ignition timing strategy, and it helps old-school racers dial in their carb at the track with which jets to install and what the tire pressures should be set at. Now, understanding barometric pressure, take this same principle and apply it to the air you’re breathing and are currently surrounded by.
I reside in Florida and thus I live at sea level. At sea level we’re at the bottom of the ocean of air above us. Being at the bottom of the ocean of air, I have all that air above me to support and as a result I suffer with the burden of carrying 14.7 lbs of air per square inch on my body as that is the standard pressure of air at sea level. Barometric pressure is the pressure exerted by the weight of air over a given area and it varies with altitude. The higher you go, the less air you have to carry and therefore the less pressure. My buddy lives in Aspen, Colorado and being 8,000’ higher from the bottom of the ocean of air, he only has to deal with 10.9 lbs per inch, or more than 25% less than I must suffer.
In the following picture you’ll see what the effects of air pressure and altitude can do. Check out the bottle…

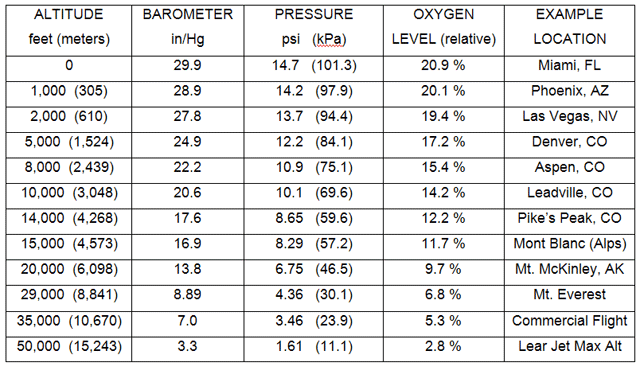
This picture was taken immediately after landing from a quick plane ride. Always being on the job and never missing an opportunity to do a good turn for you folks, I brought aboard a bottle of soda, drank it once I reached an altitude of 6500 feet, and then capped the bottle. You can see what happened to the bottle when it reached sea level. This is a true and very real physical representation of the difference between 11.5 psi (inside the bottle) and 14.7 psi (outside the bottle). Imagine the horsepower difference of an engine running at the two altitudes. To make up that lost air pressure in an engine running in Aspen, CO, you’d need a turbo producing at least 4 psi of boost just to make the same power as the guy living at sea level (all factors being equal, which they aren’t, but more on that later). If you’re driving up to the top of Pike’s Peak, you’d have only 56% of the power you made at sea level as there’s only 8.3 psi of pressure up there. Boost? You’d need at least 6.5 psi if your intercooler and compressor were perfect and ideal. Real world, with efficiency of about 70%, you need at least 9 psi of boost to get the same power from the engine on top of Pike’s Peak that you had normally-aspirated at sea-level (again, assuming everything is equal and perfect, which it isn’t). The table below should give you a good idea of the relationship between elevation and pressure change as well as how much oxygen is available to burn fuel.
Understanding barometric pressure, we can now move on to Density Altitude. Density altitude is an expression of the ambient air properties relative to the pressure altitude and adjusted for non-standard temperature. An increase in air temperature results in an increase in density altitude as higher temperature air is less dense and therefore weighs less (this is where the clever folks connect the dots and realize that boost pressure is only as effective as its temperature; more boost pressure doesn’t necessarily mean more air mass: it’s temperature dependent). This makes it comparable to air at a higher altitude as measured at standard temperature as defined by the International Standard Atmosphere standard. Higher humidity also results in an increase in density altitude as water vapor is lighter than air. Thus, in hot and humid conditions, the density altitude at a particular location may be significantly higher than the true altitude of that location. Pilots need to understand this as the performance of an airplane, both engine performance and the aerodynamic lift generated by the wings and propeller(s), is affected by increased density altitude. The higher the density altitude, the more runway the plane will need as the engine can’t accelerate the plane as quickly (it won’t make as much power) and a higher velocity will need to be reached to get enough lift from the wings to get the plane off the ground. Since the propeller is an airfoil, it too will have less “lift” and will therefore produce less thrust at a given RPM. The pilot will also have to adjust the engine mixture control to lean the engine fuel mixture out slightly to compensate for the increased density altitude. Similarly, with higher density altitude you will need to re-jet your carb to get the ideal fuel/air mixture. The higher the density altitude, the leaner the jetting will need to be to maintain the proper air/fuel ratio. If you’re running EFI and you’ve tuned and calibrated it properly, you probably won’t have to do much unless you’re running a Speed-Density system (SD). If you are, you may need to make minor adjustments to ensure the correct fuel mixture. To give you an example of the above, consider the following three scenarios:
Scenario 1:
If you are at an altitude of 1000’ and the temperature of the air is 45°F (7°C), the humidity is 32% and the barometer reads 29.0 inHg, you will be running at a lower density altitude than if you’re running at sea-level on a 90°F day (32°C) with 80% humidity and barometer reading of 29.9 inHg. The density altitude of the former would be approximately 1,400’ while the density altitude of the latter would be approximately 2,500 feet. The guy at the higher ground-level altitude will make more power from the same engine under these circumstances as he’s running at a lower density-altitude.
Scenario 2:
Let’s go back to my buddy in Aspen, CO. As I write this, the weather in his area is as follows:
Temp: 80°F; Barometer: 30.2 inHg; Humidity: 21%; Dew Point: 36°F
Density Altitude = ~ 10,880 feet.
If the temperature were to drop to 45°F but everything else remained the same, the new density altitude would be ~ 8,659 feet.
Scenario 3:
The weather here in Miami at this moment is as follows:
Temp: 90°F; Barometer: 29.7 inHg; Humidity: 51%; Dew Point: 69°F
Density Altitude = ~ 2,535
If the temperature here drops to 45°F but all else stays the same, the new density altitude would be ~ -616 feet (yes, negative! Below sea level conditions)
So, you can now see how much the temperature, humidity and barometric pressure can affect performance. If you’re tuning a carb, you’ve got to keep your little box of jets handy when you run from track to track and get yourself a good weather station if you’re going for the trophy. You’re best off keeping a log of the car setup that’s indexed according to the weather readings and track temperatures. In airplanes, the Pilot’s Operating Handbook (POH) has Performance Tables that allow the pilot to calculate runway take-off and landing lengths. Serious racers create and keep similar data handy for their purposes so that they can set their car up quicker for any given track condition.
If you run a modern EFI system that’s equipped with both a mass airflow sensor and a map sensor, you don’t have to worry about your engine if it was initially tuned properly. Just dial in your suspension and sit back and enjoy watching your neighbor tweak his carb. If you run a speed-density system, stay on the ball and make any adjustments necessary as indicated MAP pressure won’t truly reflect the density altitude, no matter how accurate the ECM, as most of these systems don’t take humidity into account. Even if the ECM gets a good reading from the MAP and intake air temp sensor (IAT), the lack of a good measure of humidity with an SD system betrays the accuracy and that is why the guys running a MAF can sit and have a beer while the SD guys are tinkering. Getting familiar with using a weather station and tuning accurately for different density altitudes will keep the pressure on your competitors and put the winning money in your pocket.
Behind the Scenes: Are you new to aviation? If so, check out the pictures below to take a behind-the-scenes look at our little R&D trip and to learn more about the aviation side as it relates to the above article.

“Aviate, navigate, communicate.” And mind the fuel mixture. A running engine makes flying fun.





